Motile Aeromonad Infection
جهت مشاهده متن کامل مقاله اینجا کلیک کنید
Motile Aeromonad Infection (MAI; Motile Aeromonas
Septicemia [MAS] Red Sore)
Introduction
Originally the genus Aeromonas was maintained within the Vibrionaceae, but there have been persuasive arguments for the erection of a separate family and this is now generally accepted (Aeromonadaceae)
It comprises Gram - negative bacilli measuring 0.3 – 1.0 × 1.0 – 1.5μm. Except for one species, Aeromonas salmonicida, they are motile by means of a single polar flagellum
They are nonsporulating, facultative anaerobes, resistant to the vibriostat 0/129
Motile aeromonad infection (MAI) is probably the most common bacterial disease of freshwater fish. All freshwater fish are probably susceptible
Motile aeromonads may also inhabit brackish water but decrease in prevalence with increasing salinity, although they are occasionally isolated from diseased marine fish
By far the most important fish pathogen is A. hydrophila (syn. A. liquefaciens, A. formicans), and members of this group are often referred to as the A. hydrophila complex
Many other Aeromonas species have been taxonomically identified, but only a few aeromonads have been considered fish pathogens (e.g., Aeromonas allosaccharophila, A. sobria, A. jandaei, A. bestiarum, A. caviae and A. veronii)
The first report of the isolation of what is presumed to have been A. hydrophila was by Sanarelli (1891)
- Aeromonas hydrophila is a mesophilic, motile, fermentative Gram negative rod which produces catalase and cytochrome oxidase
- Motile aeromonads are also commonly isolated from the mucosal surfaces and internal organs (intestinal flora) of clinically healthy fish
- Predisposing risk factors include: high temperatures (when the water temperature is above 10ᵒC), overcrowding, organic pollution, and hypoxia
- Motile aeromonads often invade skin wounds, commonly with water molds, or ectoparasites. Aeromonas hydrophila is often associated with the protozoan Epistylis in causing widespread epidemic skin lesions known as red-sore disease
Culture
- A. hydrophila is a nonfastidious organism which can be readily isolated from kidney or blood of affected fish on ordinary nutrient media
- White to buff, circular, convex colonies are formed within 24 hours at 22 – 28°C
- Rimler – Shotts (R – S) agar, a selective medium containing novobiocin, has been found useful for the isolation and presumptive identification of A. hydrophila from material likely to be contaminated with other bacteria
- It is an oxidase - positive organism resistant to the vibriostatic agent 0/129. G + C% of DNA is 57 – 63%
Epizootiology
- hydrophila is perhaps the most important cause of severe outbreaks of disease in pond-cultured and wild fresh-water fishes
- It is usually associated with the development of bacterial haemorrhagic septicaemia (known as aeromonad septicaemia or red pest), in fishes which are under stress for some other reason
- Due to the ubiquitous distribution of the organism, fish can be at risk at any time, although severe epizootics in carp occur most frequently in spring when water temperatures are rising and the fish are stressed from poor overwintering conditions
- Acute outbreaks may also be related to handling or crowding stress in water with elevated temperatures and the organism has been associated with spawning mortality in salmonids
- It is also involved in the heavy mortalities in epizootics of aphanomycete (water mould)– associated haemorrhagic septicaemia which occur in rice field fishes in South and South - East Asia
- A. hydrophila is not commonly associated with disease in marine fish but it has caused losses in Chrysophrys (snapper) major in South - East Asia by extension from skin wounds to the spinal cord
- Apart from fish, A. hydrophila infections have been documented in frogs, alligators, turtles, shrimp and man
- History: Acute to chronic morbidity/mortality
- Physical Examination: Red areas on body; skin ulcers; depression; exophthalmos; peritonitis (swollen abdomen)
Clinical Pathology
- Affected fish are usually under stress from some other factor and show darkening in colour, with large red irregular haemorrhages on the body surface and base of fins and ascites
- The haemorrhages on the skin surface may ulcerate to form shallow necrotic lesions
- Internal organs, when necropsied, are seen to be congested, with haemorrhages over the viscera
- Incision of the kidney and the swollen spleen usually results in the semifluid contents dripping out (hence the previous name A. liquefaciens)
Histopathology
- Histopathology is indistinguishable from that of pseudomonad infection, with the renal and splenic haemapoietic tissue reduced and the remaining cells necrotic
- The intestinal mucous membrane is usually necrotic and sloughed into the lumen
- Focal necrosis is found in cardiac muscle, liver, gonad and pancreas
- The skin lesions begin as severe oedema of the dermis and hyperaemia of the stratum reticulare, leading to spongiosis and ulceration of the epidermis followed by extensive haemorrhagic necrosis down to the level of the muscle, but usually the lesions are more superficial than those of vibriosis
Diagnosis
Method of Diagnosis:
- Culture of large numbers of motile aeromonad bacteria from typical skin and/or internal lesions
- Definitive diagnosis of motile aeromonad infection requires biochemical identification of clinically significant numbers of the suspect bacterium in target tissues, with attendant clinical signs
- It is important to be certain that this is the primary infectious cause of the problem. Motile Aeromonas spp. are frequent secondary invaders, following channel catfish virus, Rhabdovirus carpio, Aeromonas salmonicida, or other infections
- Kidney is probably the best organ for isolation; lesions should also be sampled. A culture of four to six fish is advisable to confirm the diagnosis
Treatment
- 1. Eliminate primary cause
- Appropriate antibiotic (Oxytetracycline, nifurpirinol, Sulfadimethoxin-ormetoprim)
Environmental improvement, especially reduction of organic pollutant levels and temperature, where possible, and also removal of dead and dying fishes, is a considerable aid to reduction of losses
The condition can usually be controlled by treatment with antibiotics or potentiated sulphonamides, but since affected fish are usually anorexic, parenteral treatment may be necessary, a stressor in itself, as well as improvement in environmental conditions
Aeromonas sobria
Isolated occasionally from haemorrhagic conditions of Indian carps in culture and as a secondary complicating infection in outbreaks of epizootic ulcerative syndrome, and trout in Sweden and France
Habitat:
Pond muds and the aquatic environment in general, especially in tropical waters
Morphology:
Typical Gram negative, mono-trichous bacillus with rounded ends. Size is usually 0.3 – 1.0 × 1.0 – 1.5μm
Culture:
A. sobria is very similar to hydrophila in culture. Principal distinguishing features are that it does not hydrolyse aesculin, cannot grow in KCN broth or utilise L-histidine, L-arginine or L-arabinose and does not ferment salicin
Epizootiology/Clinical Pathology/Treatment
sobria is less frequently isolated from outbreaks of haemorrhagic septicaemia than A. hydrophila but on occasion it has been associated with serious losses in specific epizootics, especially in South and South East Asia, from which it has been isolated in pure culture from moribund Fish
It has also been associated with serious outbreaks in farmed perch
Clinical pathology:
The clinical pathology of fish affected by Aeromonas sobria is indistinguishable from that of A. hydrophila
Treatment:
As for A. hydrophila
Fig 1. A female brown trout with large haemorrhagic lesion due to Aeromonas hydrophila infection. The lesion has developed into a prolapse of the rectum with secondary Saprolegnia infection.
Fig 2. Chronic Aeromonas sobria infection in
fin of a carp
Fig 3. A. Aeromonas hydrophila skin infection. Channel catfish with shallow ulcer.
- B. Channel catfish with Aeromonas hydrophila Note the extensive, relatively deep skin
ulcer.
C. Rainbow trout with motile Aeromonas
Note the reddened, swollen vent.
(A photograph courtesy of A. Mitchell; C photograph courtesy of R. Roberts.)
Fig 4. Severe distention and accumulation of ascites in the abdomen of a goldfish (Carassius auratus) caused by Aeromonas hydrophila. Also note the “washboard” effect of the dermis caused by the
protrusion of scales from the body surface.
Fig 5. Scale protrusion on a carp (Cyprinus carpio) caused by Aeromonas hydrophila.
Fig 6. Growth of yellow colonies that are charcacteristic of Aeromonas hydrophila after incubation on Rimler-Shotts agar.
Fig 7. The appearance of the brown or red spot lesions on the skins of the carp, from which A. hydrophila was isolated.
Fig 8. Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus)
a.Darkness in skin with fin rot in caudal and dorsal fins (arrows).
b.Hyperemia at the bases of the pectoral fin (arrow).
c.Large haemorrhagic foci on the parietal surface of the liver (arrows).
d.Greyish white foci on liver (arrows) and enlarged gall bladder filled with emerald-green secretion.
Fig 9. Experience of fish mortalities caused by epidemic clonal strains of Aeromonas hydrophila carrying a lysogenic bacteriophage. A. hydrophila have been reported in channel catfish (Ictalurus punctatus).
Internal signs include generalized hyperemia and petechiation, renomegaly, splenomegaly, mottled livers, cloudy and bloody ascites, and flaccid intestines with intramural hemorrhage.
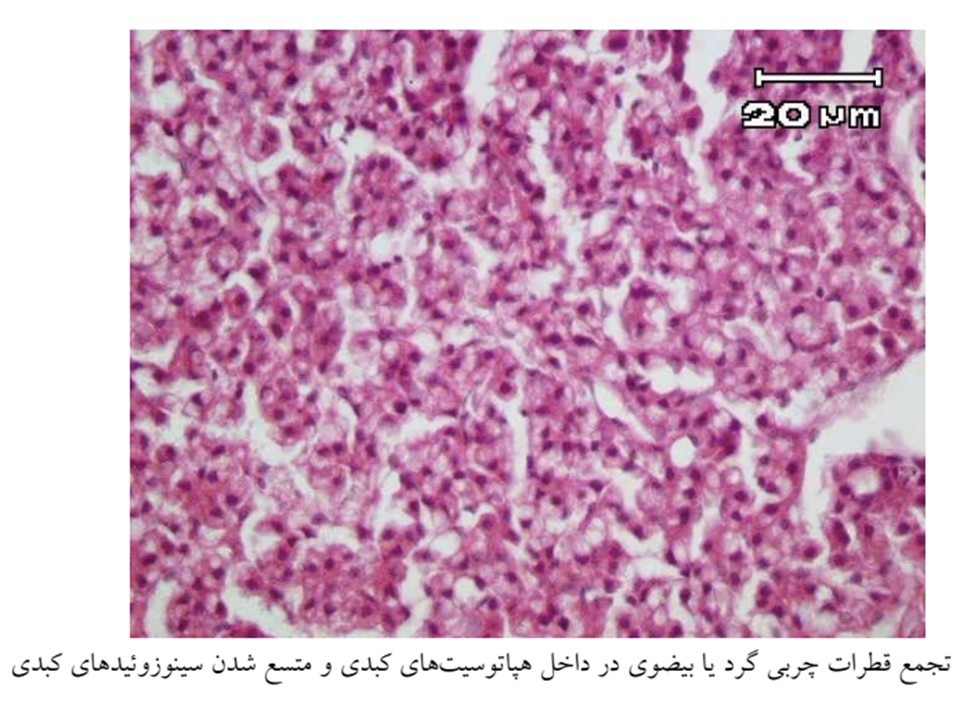
Fig 10. Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus), Liver.
Haemorrhage in liver (E2; HE, 40x).
Degenerative changes in liver (thin arrow) and necrosis of pancreatic cells (thick arrow) (D4; HE, 400x).
Diffuse lipidosis of liver (thick arrow) and hyperemia (thin arrow) (C5; HE, 100x).
Focal necrosis of hepatocytes (thin arrow) and lymphocyte infiltration (thick arrow) (D7; HE ,200x).
Fig 11. Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus).
a. Severe hyperemia in kidney (thick arrow) and degenerative changes in tubule epithels (thin arrow) (C2; HE, 200x).
b. Haemorrhage in kidney (thin arrow) and necrosis at tubule epithels (thick arrow) (E2; HE, 400x).
c. Pericardial haemorrhage (arrow) (C2; HE, 100x).
d. Lymphocyte infiltration between the cardiac muscle fibres (arrow) (B7; HE, 400x).
Fig 12. Pathological changes in lamella tissues owed to different level of A. hydrophila in freshwater Crayfish (Astacus leptodactylus).
A; Infected gill lamella (3×108 CFU ml-1) 48 h after exposing to A. hydrophila; Severe cell necrosis and hemocyte aggregation (H&E ×440).
B and C: Infected gill lamella in the treatment 3×106(B) and 3×104 CFU ml-1 (C) 48 hours after exposure time; Mild necrosis and hemocyte aggregation (H&E× 880),
D: Infected gill lamella (without of bacteria A. hydrophila as control), 48 hours after exposure time;
Normal cell appearances were observed with no any changes (H&E ×880).
Fig 13. Pathological changes in haepatopancreas tissues owed to different level of A. hydrophila in Crayfish.
E; Infected haepatopancreas of Crayfish in treatment 3×108 CFU ml-1 A. hydrophila;
massive hemocytes aggregation are infiltrated (long arrow) and many of cells shows pyknosis (short arrows) (H&E×880 ).
F and G; Infected haepatopancreas of Crayfish in 3×106 CFU ml-1(F) and 3×104
CFU ml-1(G);
hemocyte aggregation (long arrow) and pyknotic nuclei (small arrow) were observed. (H&E ×880).
H; No changes were observed in cells of haepatopancreas in control group
(H&E×440).
Fig 14. Myocardium changes owed to exposing to the bacteria in freshwater Crayfish (Astacus leptodactylus).
I; Infiltrated and aggregated hemocytes in the myocardium in Crayfish exposed to 3×108 CFU ml-1 A.hydrophil (H&E×880 ).
J; Normal heart tissue in control group (without of bacteria A.hydrophila) (H&E ×880).
Fig 15. digestive tract changes owed to exposing to the bacteria in freshwater Crayfish (Astacus leptodactylus).
K; Hemocyte infiltration in digestive system in treatment 3×108 CFU ml-1 A.hydrophila (H&E×880).
L; Normal digestive tract tissue in control group (without of bacteria A.hydrophila) (H&E×440).
Fig 16. Aeromonas hydrophila: Antimicrobial Susceptibility and Histopathology of Isolates from Diseased Catfish, Clarias gariepinus (Burchell).
A. Yellow foci on the parietal surface of the liver [1] Greencolor of liver [2] and enlarged gall bladder filled with emerald-green secretion [3]
B. Hemorrhage of the skin.
C. Ascites.
Fig 18. Aeromonas hydrophila: Antimicrobial Susceptibility and Histopathology of Isolates from Diseased Catfish, Clarias gariepinus (Burchell).
Histopathological section of
A: Skin; (a) necrosis in the dermal layer (b) hypertrophy in immersion group with (H&E stain100X).
B: Gill; (a) hyperplasia in the secondary lamellae (b) leukocytic infiltration (c) dilation of the central venous sinus (H&E stain100X)
C: Kidney; (a) degenerative changes
in glomerular epithelium (b) inflammatory cells (H&E stain 100X)
D: liver; (a) hepatocytes vacuolar degeneration (b) inflammatory cells (H&E stain 100X)
E:Muscles; (a) mild edema (b) focal hyaline degeneration
F: Spleen; hyperplasia in the lymph follicles.
Fig 19. Histopathology of diseased mud loach, Misgurnus mizolepis with Aeromonas sobria infection.
(A) Gill showing epithelial lifting and hyperplasia of epithelial cells at the basal portions.
(B) Operculum showing damaged epithelial and club cells, and haemorrhage in the underlying dermal loose connective tissues.
(C) Liver displaying hepatocellular vacuolar degeneration and congestion in sinusoids.
(D) Hepatic cells
presented karyorrhexis, karyolysis, karyopyknosis and hypochromatosis of the nuclear membrane.
Fig 20. Histopathology of diseased mud loach, Misgurnus mizolepis with Aeromonas sobria infection
(E) Kidney showing renal
Parenchymal haemorrhages, tubularr necrosis and peritubular haemorrhages. (F) Interstitial lymphocyte infiltration (arrow head) and
haemosiderin granules (arrow) exhibiting in the parenchyma of the kidney.
(G) Spleen showing haemorrhage, deposition of
haemosiderin (arrow) and destruction of sheathed tissue.
(H) Raised lesion on the head displaying epidermal exfoliation and haemorrhages and edema in the underlying dermal loose connective tissue, (scale bars, 100 µm).
References
I.A Colour Atlas of Salmonid Diseases, David W. Bruno, et al (2013)
II.Fish Disease Diagnosis and Treatment, Edward J. Noga, (2010)
III.Fish Pathology, Ronald J. Roberts, (2012)